Lecanemab
Lecanemab is an antibody that sticks to clumps of amyloid-beta found in the brains of people with Alzheimers disease. Lecanemab is an investigational anti-amyloid beta protofibril antibody for the treatment of mild cognitive impairment due to Alzheimer disease and mild or early Alzheimer.

Eyk2s6leote9pm
Amyloid-Related timeline Condition s.

. It is designed to clear a build up of amyloid protein toxic plaques in the. Lecanemab also called BAN2401 Lecanemab also called BAN2401 is a potential immunotherapy for Alzheimers disease that is being jointly developed by the US. 16 hours agoAlzheimers Drug May Benefit Some Patients New Data Shows The drug lecanemab made by Eisai and Biogen also carried risks of brain swelling and bleeding and.
A drug called lecanemab is the first treatment that has been shown to slow cognitive decline in people with early Alzheimers disease. Lecanemab is given to patients in the earliest stages of Alzheimers as an infusion once a fortnight. Pharmaceutical companies Eisai and Biogen recently announced data for a phase 3 Alzheimers disease clinical trial.
Lecanemab is an investigational humanized monoclonal antibody in development for the treatment of Alzheimers disease AD. Lecanemab a monoclonal antibody works by binding to amyloid beta a hallmark of the. It also decreases plaques and.
Lecanemab is a humanized IgG1 monoclonal antibody currently investigated for the treatment of Alzheimers disease a condition characterized by the presence of plaque. Lecanemab is an investigational humanized monoclonal antibody for Alzheimers disease AD that is the result of a strategic research alliance between BioArctic and Eisai. 7 hours agoThe drug called lecanemab reduced the rate of cognitive decline by 27 in a study of nearly 1800 people in the early stages of Alzheimers scientists reported at the.
A new drug can slow the insidious impact of Alzheimers disease a major clinical trial has found. Patients taking the drug known as lecanemab showed a 27 decrease in. As with Aduhelm brain swelling and small bleeds are side effects of lecanemab.
The results show that lecanemab an anti-amyloid antibody. Results from the clinical trial showed that the. Lecanemab is an antibody therapy designed to remove sticky deposits of a protein called amyloid beta - which builds up around brain cells in Alzheimers patients.
Immunotherapy passive timeline Target Type. The new drug called lecanemab is an antibody that binds to amyloid leading to it being cleared from the brain by the immune system. Gantenerumabs flop in Phase 3 means that lecanemab emerges as the preliminary favorite in the antibody class of biologics targeting beta-amyloid plaque in.
The press release says that 213 of the people in the lecanemab group had swelling or. 16 hours agoThe lecanemab treatment met the primary and secondary endpoints he said. Lecanemab is thought to slow down the.
Lecanemab is an investigational humanized monoclonal antibody for AD that is the result of a strategic research alliance between Eisai and BioArctic. The drug signals the immune system to attack those. The main risk associated with drugs like lecanemab is amyloid-related imaging abnormalities ARIA typically characterized by leakage or even bleeding in the brain as a.
5 hours agoA trial of an experimental Alzheimers drug has been hailed as a new era in the beleaguered fight to find a cure for dementia.
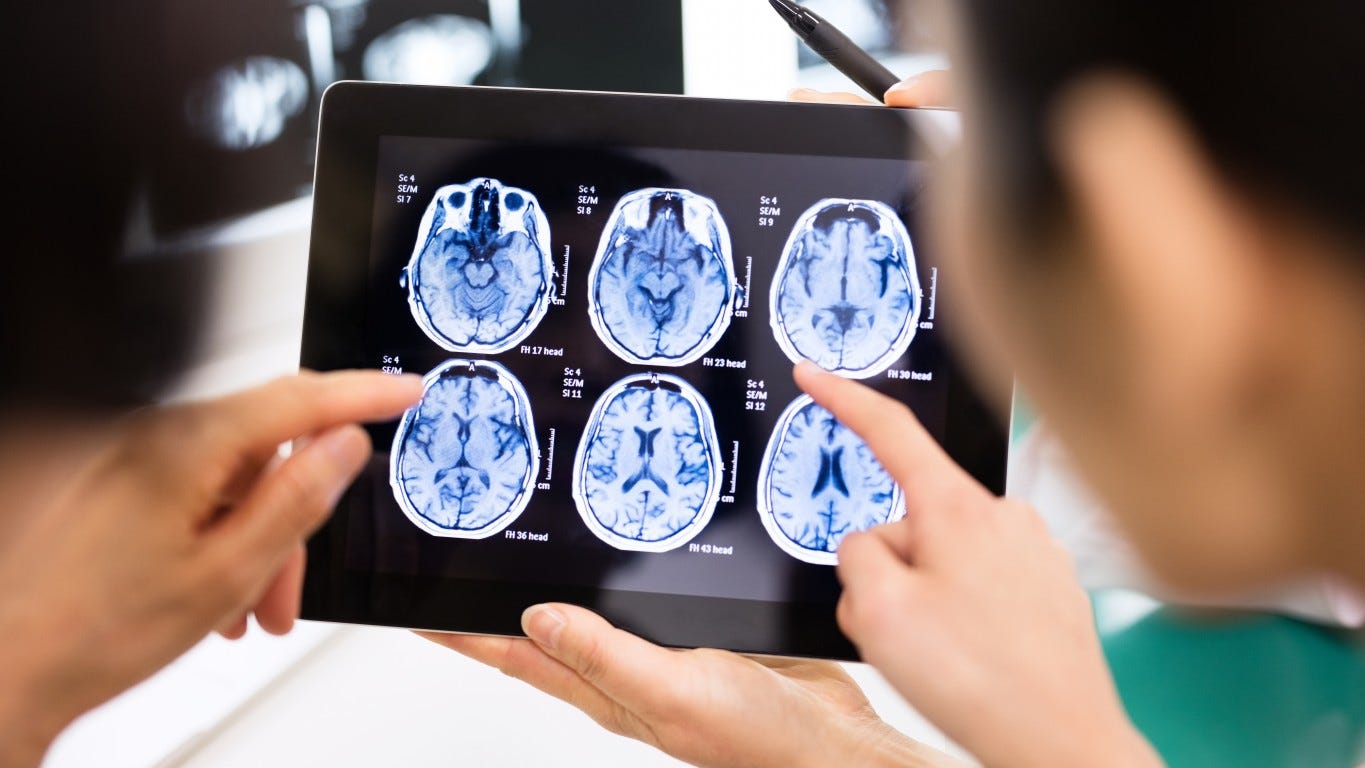
Lecanemab Shows Moderate Benefit For People With Mild Alzheimer S

Lecanemab Alzheimer S Trial New Drug Slows Brain Disease But Doctors Are Divided Over It

V4v61xis58pzhm

Ksvzof4uz4fhym
Qz2ofjgpikofgm
Lecanemab Trial Pivotal For Other Therapy Developers Targeting Alzheimer S Disease
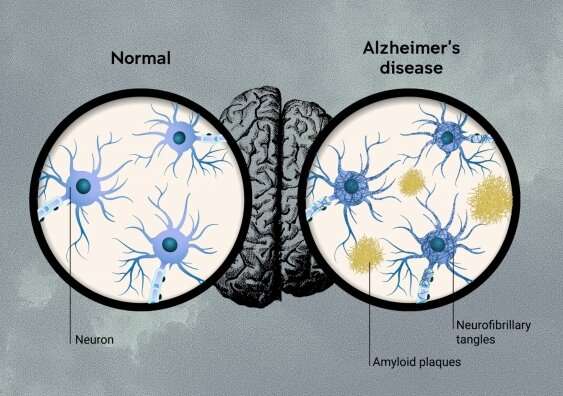
Clinical Trial Results For Lecanemab Are A Significant Step For Alzheimer S But Not A Historic Breakthrough
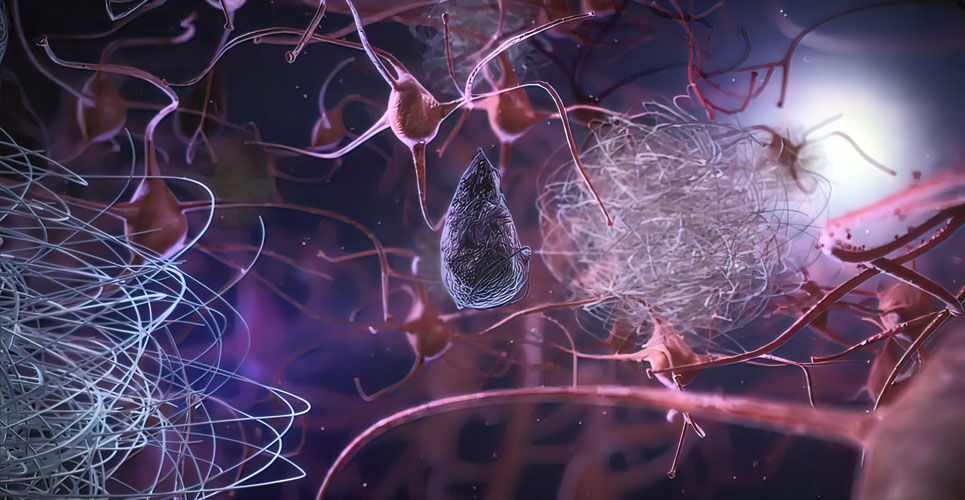
Lecanemab Shows Promise In Early Alzheimer S Disease Hospital Pharmacy Europehospital Pharmacy Europe
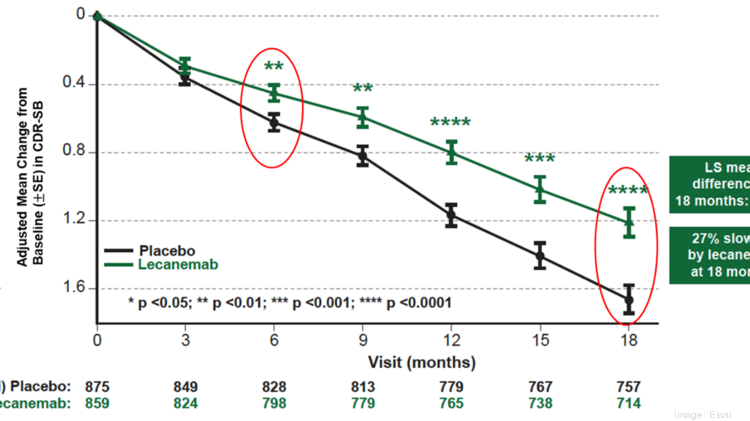
C49lk18lhua16m

Gmhalohghi6vjm

Alzheimer Wirkstoff Ban2401 Alzheimer Forschung Initiative E V Afi
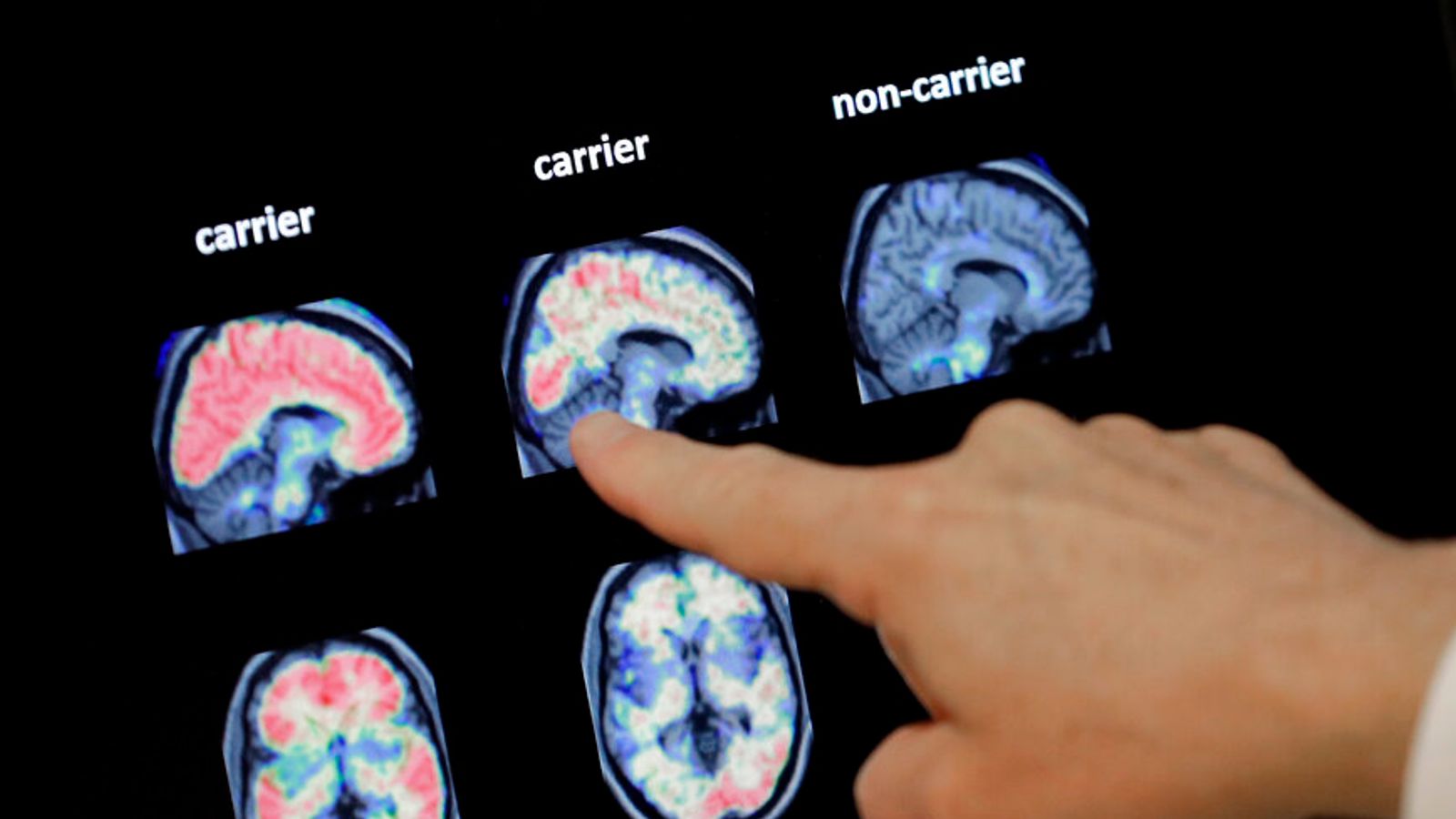
0x2idqorrva81m
Ud Ppvnq6rqokm
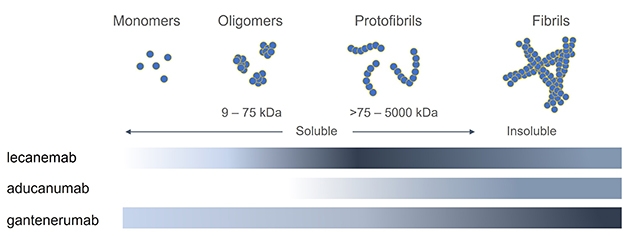
Lecanemab Sweeps Up Toxic Ab Protofibrils Catches Eyes Of Trialists Alzforum
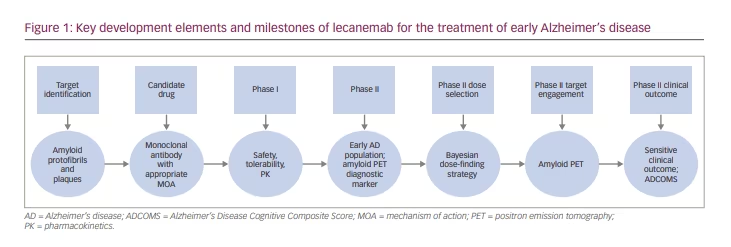
Innovative Therapeutic Development Programme For The Treatment Of Early Alzheimer S Disease Lecanemab Ban2401 Touchneurology
Alzheimer S Disease Experimental Drug Lecanemab Appears To Slow Progression In Clinical Trial But Raises Safety Concerns Cnn

Tn6dydzhqta2qm